National Manufacturing Institute Scotland
Case Studies
NMIS boosts development of a promising new technology for robotic surgery
Background
Nami Surgical is a high-growth spin-out from the University of Glasgow that develops high-performance miniaturised ultrasonic scalpels for robotic assisted surgery.
The team was awarded Research & Technology Organisation growth funding from Innovate UK EDGE in August 2022. The grant was used to enlist the support of NMIS to help progress their latest prototype to the next technology readiness level.
Challenge
It is critical for Nami Surgical to design and manufacture products that meet the medical-grade high standards of their clients - the manufacturers of surgical robots. The scalpels are co-developed with robotic surgeons to ensure compatibility with wristed robotic joints, enabling a faster and safer surgery.
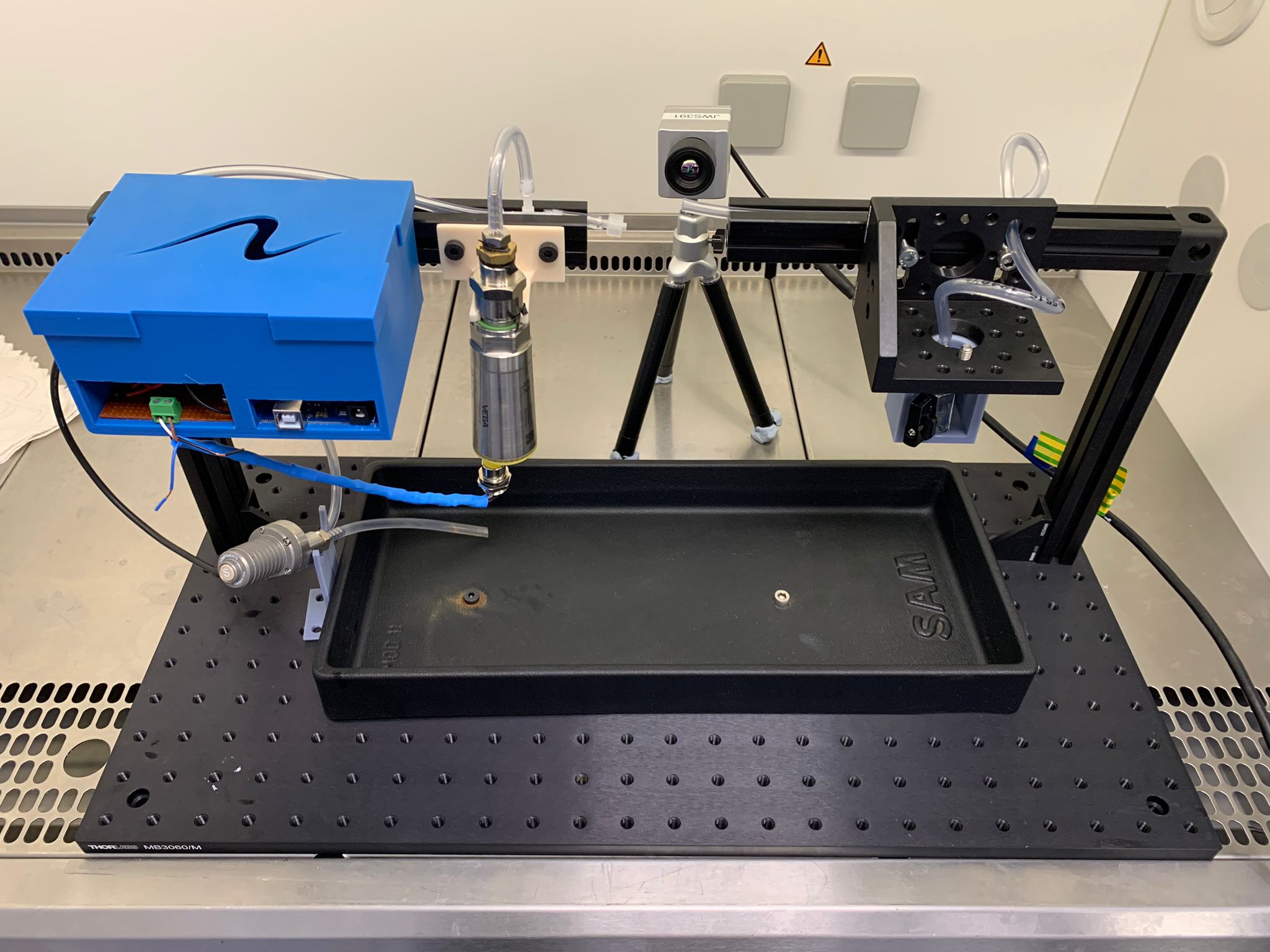
In order to bring the latest iteration of their scalpel to market, the team required external expertise to assist them in developing a bespoke rig which allows them to conduct a vessel sealing burst pressure test (VSBPT) on their prototype.
What did NMIS do?
NMIS’ SME engagement specialist and design engineering team worked in collaboration with Nami Surgical to first gain an in-depth understanding of the technical and commercial requirements.
The experts at NMIS then recommended a modular design for the test rig, allowing the prototype to be developed on-site at Nami Surgical’s 3D printing facility. This would enable the in-house production of all bespoke parts for the rig. They also shared advice and guidance on components that are not suitable for 3D printing but can be purchased off-the-shelf.
With health and safety in mind, and as the VSBPT is a wet process, NMIS ensured the voltage limit of the rig’s electric equipment adheres to best practice guidelines from the Health and Safety Executive’s electricity at work regulations.
The NMIS team also carried out a study to identify the maximum applied force of the rig, and subsequently suggested suitable off-the-shelf clamping techniques that align with the force limit.
Throughout the development stringent standards of the highly regulated medical technology sector were considered by the engineers of NMIS, who drew on their expertise and experience of healthcare applications.
Nico Fenu, CEO and founder of Nami Surgical, said:
NMIS played a pivotal role in shaping our success. By helping us to successfully build and validate our VSPBT testing rig, we were able to rigorously test our prototypes in accordance with industry standards. This achievement has instilled confidence in both customers and investors, demonstrating the reliability and quality of Nami Surgical’s prototypes.”
Business Impact
NMIS’s advice and guidance provided the team at Nami Surgical with the knowledge to take their prototype to the next stage of development.
By suggesting that the components of the test rig could be manufactured in-house, NMIS enabled Nami Surgical to minimise costs during the product development stage. They also highlighted the parts of the rig that could be purchased off-the-shelf, an additional cost saving approach.
The results obtained through this experimental setup have been instrumental in Nami Surgical’s business development, with the company on track to secure £2.5 million in investment at the beginning of 2024. Nami has successfully engaged with its first commercial partners, setting the stage to bring this technology to the market within the next 24 months.
Furthermore, the test rig also plays a role in delivering social impact and advantages for surgical patients. This technology will enable surgeons to employ more appropriate instruments in robotic surgery, harness the potential of ultrasonics in robotics, and, ultimately, broaden the utilisation of robotic surgery in critical procedures that necessitate ultrasonic technology.
